Interaction of Metal-based Drugs with Proteins
Metal ions are essential for the function of certain enzymes. The acquisition and regulation of metal ions in cells is governed by a sophisticated array of “transport proteins” or “chaperone proteins” (Figure 1).
In collaboration with Prof. Tony Wedd and Dr. Zhiguang Xiao in the Bio21 Institute we are investigating the interactions of these transport proteins with metal-based drugs. In particular we are interested in the interactions of the anti-cancer drug cis-platin and copper radiopharmaceuticals.
Our aim is to gain insight into the molecular nature of these important biochemical interactions and this may help to design drugs with fewer side effects.
The molecular probes needed are provided by techniques such as NMR, ESR, MS, fluorescence, X-ray crystallography and electrochemistry.
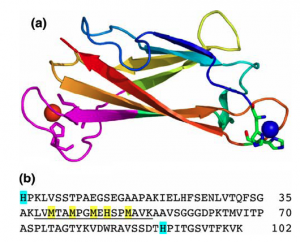
Top: Molecular structure of CuICuII-CopC, b) Amino acid sequence of CopC showing ligands for CuII and possible ligands for CuI highlighted in cyan and yellow, respectively. Tryptic digestion produces the underlined peptide fragment of residues 38–54 that retains the entire CuI binding loop (highlighted in pink in the structure). This loop provides ligands for the Pt centre.
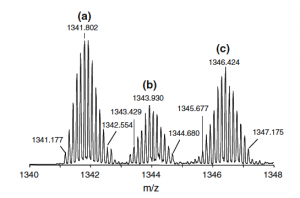
Bottom: ESI-FTICR mass spectrum of the 8+ charge state region of the 1:1 products from a reaction of cisplatin and apo-CopC, (a) [Pt–CopC]8+, (b) [(NH3)Pt–CopC]8+, (c) [ClPt–CopC]8+.